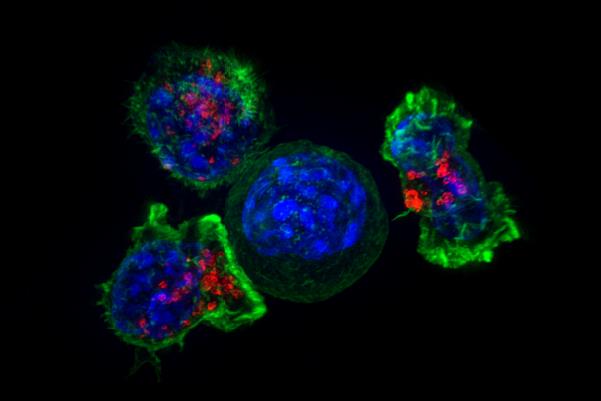
Clinical trials have shown that a new immunotherapy can survive patients with advanced head and neck cancer for more than 3 years December 17, 2018 Source: Ministry of Science and Technology Killer T cells surround a cancer cell. Image source: NIH A major new clinical trial reports that a new immunotherapy can greatly extend the lives of some patients with advanced head and neck cancer, some of whom can live for three years or more. In general, the Pembrolizumab drug has a significant effect on patients, with 37% of patients receiving this drug surviving for one year or longer, compared with 26.5% of patients receiving conventional therapy. . But in people who responded to pabolizumab, the results were particularly exciting, with a median survival time of 18.4 months, compared with a median time of 5 months for conventional therapy. This large international clinical trial is led by the British Cancer Institute and the Royal Marsden NHS Foundation, with 97 medical institutions from 20 countries. The trial was funded by Merck and the results have recently been published in The Lancet. The drug is being evaluated in nearly 500 patients with advanced head and neck cancers that have spread throughout the body and have developed resistance to platinum-based chemotherapy, a first-line treatment for the disease. The researchers randomized patients to 247 patients using pabolizumab and 248 patients receiving conventional therapy, such as chemotherapy or the targeted drug cetuximab (cetuximab). When chemotherapy or targeted drugs are ineffective, these advanced head and neck cancer patients have few treatment options, and they usually do not survive for more than 6 months. Among the patients who participated in the trial, the median survival time of those taking pabolizumab and those receiving conventional treatment were 8.4 months and 6.9 months, respectively. However, a small number of patients responded very well to pabolizumab, and the tumors of 36 patients partially or completely disappeared, and after the initial dose, some people still did not relapse in the next three years. Pabolizumab caused fewer serious side effects than currently approved, and 13% of patients receiving this immunotherapy experienced severe side effects, compared with 36% for conventional therapy. Researchers at the British Cancer Institute (ICR) and the Royal Marsden NHS Foundation hope that Pabolizumab will be a more sensible and friendly treatment option for patients with advanced head and neck cancer. Pablolinizumab works by releasing the "brake" for the immune system's ability to attack cancer cells, which have been approved for lung, skin and lymphoma. Professor Kevin Harrington of the ICR Biological Cancer Therapy, a consultant to the Royal Marsden NHS Foundation Trust, said: "Head and neck cancers are difficult to treat once they relapse or spread, and once other treatments fail, the patient's prognosis will be very poor. Our study shows that the immunotherapeutic drug, pabolizumab, prolongs the life of patients with advanced head and neck cancer, and that some patients have significantly improved their condition. It is a very mild drug compared to currently approved treatments. We hope that Pabolizumab can be approved for clinical use, so that patients with advanced head and neck cancer have an opportunity to prolong life and improve their quality of life. We also urgently need to figure out how to determine which patients are most likely to Benefit from the drug, because for some patients, the efficacy of pabolizumab is much better than conventional therapy." Professor Paul Workman, head of the British Cancer Institute, said: "Immune therapy has revolutionized the treatment of several types of cancer. I am very happy to see the efficacy of the new drug, pabolizumab, in the treatment of advanced head and neck cancer. The key challenge is to design effective immunotherapy for more people, so that more patients can benefit from the response to this drug as patients in this clinical trial."
Animal source Protein Peptide mainly include bovine collagen protein peptide,Yak bone collagen peptide,oyster protein peptide,sea cucumber protein peptide,fish collegan peptide etc. Different peptide has respective effect to human body. Bovine bone collagen peptide contains aspartic acid, glutamic acid, serine, histidine, glycine, threonine, arginine, alanine, tyrosine, cystine, valine, methionine, phenylalanine, isoleucine, lysine, proline.Yak bone collagen peptide is highly rich in glutamic acid, serine, histidine, glycine, alanine, tyrosine, cystine, valine, methionine, phenylalanine, isoleucine, proline. Oyster peptide is good in male reproductive function. Sea cucumber peptide can improve the immunity and the immune level of the body. Fish collagen peptide is good for anti-aging products.
bovine collagen protein peptide,Yak bone collagen peptide,oyster protein peptide,sea cucumber protein peptide,fish collegan peptide Allied Extracts Solutions , https://www.nballiedbiosolutions.com