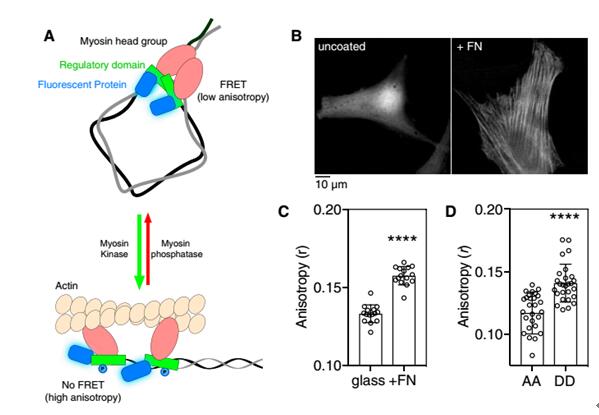
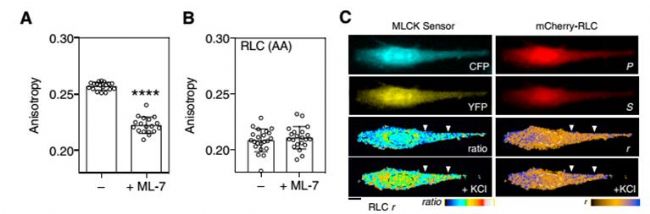
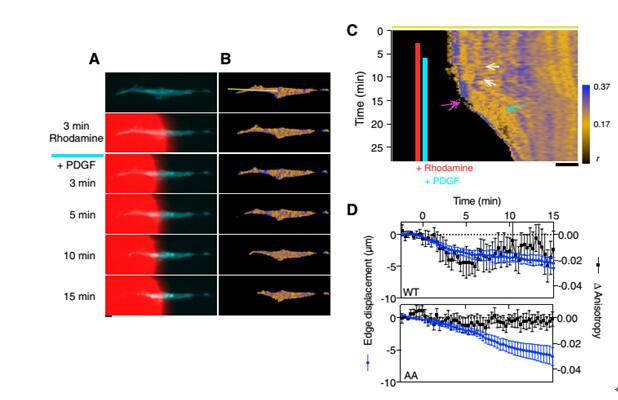
Phosphorylation of the regulated light chain (RLC) causes confluence of non-muscle myosin II (NMMII) with actin fibers, resulting in the formation and contraction of the actomyosin cytoskeleton. NMMII plays an important role in cytoskeletal dynamics, but how to accurately regulate the phosphorylation of RLC at the subcellular level remains unknown. Current research methods are not suitable for the observation of RLC phosphorylation in living cells and organisms. For example, the limitation of using two-color fluorescence resonance imaging technology is that occupying most of the available spectrum results in incompatibility with other optical sensors and phototoxicity and photobleaching. This study developed a homologous FRET method for the observation of fluorescent protein-labeled RLC phosphorylation.      As shown in Figure 1A, phosphorylation results in dissociation of the RLC dimer, attenuates FRET, and increases anisotropy. The fluorescent protein-fused RLC was transfected into fibroblasts and cultured in glass dishes and fibronectin-coated glass dishes. It was found that the anisotropy of the experimental group plated with fibronectin was significantly increased. And the anisotropy of the experimental group of RLC mutant AA (lack of phosphorylation site) was significantly reduced.  Next, mCherry-RLC was used to explore the kinetics of RLC-FRET in living cells. MLCK is capable of phosphorylating RLC, as shown in Figure 2A. After ML-7 treatment inhibits MLCK activity, the anisotropy of mCherry-RLC is significantly reduced. The mCherry-RLC was co-expressed with the MLCK FRET biosensor, and the polarization of the intracellular mCherry-RLC was also increased after activation of MLCK with KCL.      Next, the researchers needed to perform single-cell stimulation to observe the dynamic process of myosin phosphorylation at the subcellular level. Before cell shrinkage, extracellular overhangs contain stable, highly anisotropic phosphorylation regions (Fig. 3B), followed by PDGF stimulation of extracellular overhangs using Biopen, and Biopen, a single-cell microfluidic drug delivery system from Swedish company Fluicell, using high-end microfluidics, fluid can be precisely controlled in the coverage under a microscope, as shown in the red rhodamine dye is the area stimulated area coverage of this experiment. First, the cytoskeletal contraction is 10-20 μm (Fig. 3C, white arrow), the RLC anisotropy of the anterior end of the contraction is increased (Fig. 3C, in front of the pink arrow), and the dephosphorylation of myosin, including the contraction front (Fig. 4C, pink) Below the arrow) and inside (Figure 3C, blue arrow). Quantitative statistics of the anisotropy changes of contractile margin myosin RLC throughout the process are shown in Figure 3D. There is no significant change in the anisotropy of the mCer3-RLC (AA) edge, and the wild type RLC anisotropy is significantly reduced. Biopen uses the surface tension formed by the flow rate of the suction stream higher than the flow rate of the injection stream to automatically form a liquid flow circuit that separates the cell culture system from the perfusion liquid through a virtual boundary to create a local flow chamber. The size of the flow chamber can be controlled in the environment surrounding the individual cells in the cultured cells or tissues. The sub-second delivery rate of 5-25nL/s enables fast switching of 4 different liquids. With our single-cell microfluidic drug delivery system, the researchers completed a single-cell level of myosin phosphorylation and established a test method that is compatible with many different fluorescent proteins and biosensors. A great tool in the field of single cell research! Biopen technical advantage ★ Accurate single cell or multicellular drug environment control molecules; ★ low drug consumption, 35μL / hole; ★ 4 kinds of solutions to switch quickly; ★ No need to clean; ★ Non-contact administration, no pollution; ★The top of the gun head is made of non-glass polymer soft material, which is not easy to break and avoid damage to cells; ★ Maximize the use of cultured cells; ★ Compatible with major brands of inverted micro imaging systems; Biopen application area ★ Enzymology; ★ electrophysiology; ★Pharmacology; ★ histopathology; ★ drug discovery; ★ biological printing; Lower Blood Sugar Plant Extract Lower Blood Sugar Plant Extract,Inonotus Obliquus Extract,Chaga Extract Powder,Chaga Mushroom Extract Powder Polysaccharide Fufeng Sinuote Biotechnology Co.,Ltd. , https://www.sntextract.com