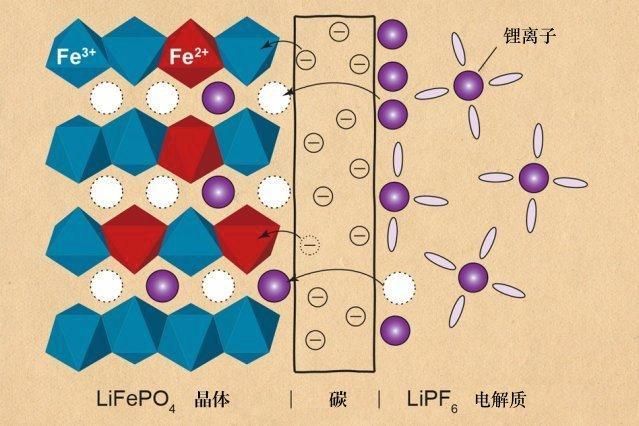
As the material basis of human activities, energy is like a hand that holds the throat of human society. Our daily life is inseparable from the use of energy. In this blue planet with limited energy resources, energy development, energy and environment, and energy storage and regeneration are issues of common concern to the whole world and all mankind. With the continuous development of science and technology, new energy technologies such as polycrystalline silicon solar cells, electric vehicles, biomass energy, etc. have sprung up in our lives, and the demand for portable energy storage devices is much larger than before, and continues Maintain exponential growth. In order to comply with this trend, the development and production of battery technology has become more diverse to meet the needs of people for the full efficacy of the battery. Small enough to carry electronic devices, large to travel, batteries are almost everywhere in our lives. Today, Xiaobian came to tell you about the university in this small battery world! Step into the interesting electrochemical world In the battery world, the first thing to mention is the use of a common lead-acid battery with a history of nearly 150 years, which is widely used in transportation, communications, electric power, military, navigation, aviation and other fields. Structurally, the electrode of a lead-acid battery is mainly made of toxic heavy metal lead and its oxide, and the electrolyte is a highly corrosive sulfuric acid solution. In the discharge state of the lead-acid battery, the main component of the positive electrode is lead dioxide, and the main component of the negative electrode is lead; in the state of charge, the main components of the positive and negative electrodes are lead sulfate. Lead-acid batteries have significant limitations in terms of durability, portability and environmental friendliness. Generally, the deep charge and deep discharge is less than 300 times, and there is memory. The life span is about two years, and after the liquid in the battery is consumed for a period of time, if the battery is found to be hot or the charging time is short, the liquid needs to be replenished; The weight of the lead-acid battery is 16~30 kg, which is large in size and difficult to carry. In addition, the battery is likely to cause environmental pollution during the production process or recycling process. In order to promote sustainable development, environmentally friendly green battery technology is becoming mainstream. The most common are alkaline and lithium batteries. Alkaline batteries are also called alkaline dry batteries, alkaline zinc-manganese batteries, and alkaline manganese batteries. They are the best battery types in the zinc-manganese battery series. They are suitable for large discharge and long-term use. Compared to lead-acid batteries, alkaline batteries have proven to be a more efficient and safe alternative in some applications because they do not contain highly toxic and corrosive ingredients. The alkaline battery adopts the opposite electrode structure as the ordinary battery, and the mixture of manganese dioxide and graphite powder is used as the positive electrode, and the zinc and other additives are the negative electrode, which increases the relative area between the positive and negative electrodes, and is high. The conductive potassium hydroxide solution replaces the ammonium chloride and zinc chloride solutions as electrolytes, allowing ions to move between the two poles. In particular, the negative electrode zinc is also changed from a sheet form to a powder form, which increases the reaction area of ​​the negative electrode, and in addition to the use of high-performance electrolytic manganese powder, electrical properties are greatly improved. The total battery reaction formula is: Zn + MnO 2 + 2H 2 O + 4OH - = Mn(OH) 4 2 - + Zn(OH) 4 2 - Alkaline batteries are successful high-capacity dry batteries and one of the most cost-effective batteries. Because it is quite leakproof, it can be used in any environment. Finally, let's take a look at the most popular lithium batteries in electronic devices. Lithium batteries can be classified into lithium metal batteries and lithium ion batteries. Due to the safety problems caused by the very active chemical properties of lithium metal, the lithium-ion batteries that are widely used today are all lithium-ion batteries, not lithium metal batteries. A lithium ion battery is a rechargeable battery. Generally, a lithium alloy metal oxide is used as a positive electrode material, graphite is a negative electrode material, and a nonaqueous electrolyte battery is used. It relies mainly on the reciprocal insertion and deintercalation of lithium ions between the positive and negative electrodes to achieve energy storage and release. Common cathode materials for lithium ion batteries are lithium cobaltate, lithium manganate, lithium nickelate, lithium iron phosphate, and the like. Here, we take an example of a lithium iron phosphate battery with better safety. The PO bond in the lithium iron phosphate crystal is stable and difficult to decompose, and does not collapse or heat like a lithium cobaltate or form a strong oxidizing substance even at a high temperature or overcharge. The battery is divided into left and right sides. On the left is a battery positive electrode composed of olivine-structured LiFePO 4 . The aluminum foil is connected to the positive electrode of the battery. The middle is a polymer separator separating the positive and negative electrodes. The lithium ion Li + can pass and the electron e - cannot pass. The right side is the negative electrode of the battery consisting of graphite, which is connected to the negative electrode of the battery by copper foil. Between the upper and lower ends of the battery is the electrolyte of the battery, the main component is lithium hexafluorophosphate LiPF 6 , and the entire battery is hermetically sealed by a metal casing. At the time of charging, Li + in the positive electrode migrates to the negative electrode through the polymer separator; at the time of discharge, Li + in the negative electrode migrates toward the positive electrode through the separator. Lithium batteries have strong durability, slow consumption, long life, no memory, and are easy to carry. Although the price is relatively expensive, it is very green and environmentally friendly. It is a clean energy storage device and a development trend in the battery industry. The moisture meter can also be related to the battery? After reading the popularity of battery knowledge above, is there a feeling of returning to the familiar chemistry class? In fact, in the battery production process, there is another indicator that plays a vital role in the performance and manufacturability of the battery. This is the moisture content of the battery. Some people will feel incredible~ In the case of an alkaline battery, the positive and negative materials of the battery are mixed into a viscous material to form and form a suitable shape to construct the battery. The viscous mixture must meet strict and precise moisture content regulations. If the moisture content is too much, the conductivity will be poor and the battery capacity will be insufficient. Conversely, if the moisture content is insufficient, the battery will be difficult to form. Many battery manufacturers in the country and around the world trust Ohaus's moisture analyzer to determine the moisture content of the battery. Below is an example of a customer who produces lithium iron phosphate batteries from East China. According to relevant experiments, the cycle performance and rate performance of lithium batteries are closely related to the moisture content of the electrode. When the moisture content of the electrode exceeds 0.06%, the cycle performance and rate performance of the battery are reduced, the discharge specific capacity is seriously attenuated, and the capacity decays by nearly 40 after 200 cycles. %, and the internal resistance of the battery increases, and the electrochemical impedance increases. At the same time, the battery pole piece will absorb moisture in the special part of the actual production, resulting in the degradation of its electrochemical performance. [1] Therefore, in the production of lithium batteries, the electrode material requires extremely strict control of moisture. The Ohaus MB 120 Moisture Analyzer is equipped with a new heating chamber design, while the precisely controlled halogen heating system provides rapid temperature rise and uniform heating. Combined with a high-precision load cell, the sample moisture test readability is 0.01%/1mg. In the battery production process, the customer only needs to sample 3~5g of the electrode material powder at a time, and select the appropriate temperature according to the characteristics of the sample for measurement, which will quickly display accurate and stable measurement results. The entire process not only greatly improves the accuracy of the measurement, but also saves time and increases productivity. Ohaus's equipment not only provides fast and reproducible results in the laboratory, but also provides a reliable daily measurement service in an industrial environment. If you have more inquiries about the determination of raw materials and finished products in industrial production, or are looking for more professional and detailed moisture meter selection guide, please call 4008-217-188, or click to enter "Request Information" and leave Related information, our professional engineers will contact you at the first time! references: [1] Niu Junting, Sun Lin, Kang Shuwen, Zhao Zhengwei, Ma Zifeng. Effect of electrode moisture on the performance of lithium iron phosphate battery[J]. Journal of Electrochemistry, 2015, 21(5): 465-470. Foot Spa Massager,Bath Foot Massager Machine,Foot Spa Bath,Bath Foot Massager With Bubble Huaian Mimir Electric Appliance Co., LTD , https://www.mimirfootbath.com