medical products Anesthesia Medical Co., Ltd. , https://www.jssinoanesthesias.com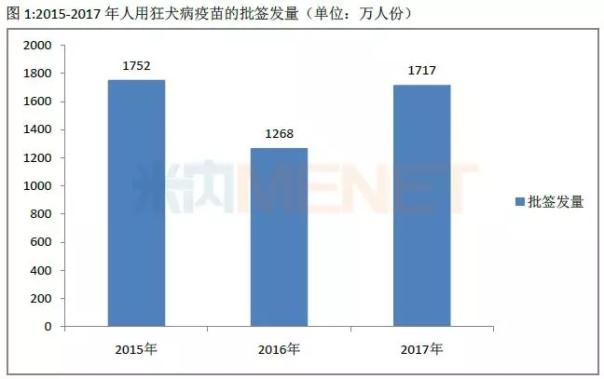

Medical Network July 30th In the past week, the crisis caused by longevity organisms has spread to the A-share market, not only the vaccine sector, but also the overall trend of the pharmaceutical bio-sector. There are data showing that as of July 23, the Shenwan Pharmaceutical Bio-integration sector as a whole It fell 3.94% and the total market value evaporated by 161.127 billion yuan. The latest news shows that the stock of Changsheng Bio-stock was put on risk warning on July 26, and the abbreviation was changed to “ST Changshengâ€, and the price limit was limited to 5%.
According to the data of the Chinese Academy of Sciences, the number of rabies vaccines issued in 2017 was close to 17.17 million, which is close to the number of batches issued in 2015. What kind of rectification will such a huge market face?
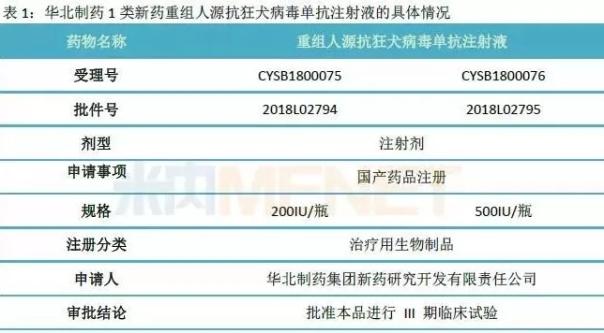
On July 20, Huabei Pharmaceutical announced that it has approved the application of Phase III clinical trial of a new class of recombinant human anti-rabies virus monoclonal antibody injection, which was independently developed by its subsidiary, Huabei Pharmaceutical Group New Drug Research and Development Co., Ltd. If it can be listed smoothly in the future, can it become a new force in the market?
The number of human rabies vaccines issued is more than 10 million
(Note: According to the data of the Chinese Academy of Sciences)
(Note: According to the data of the Chinese Academy of Sciences)
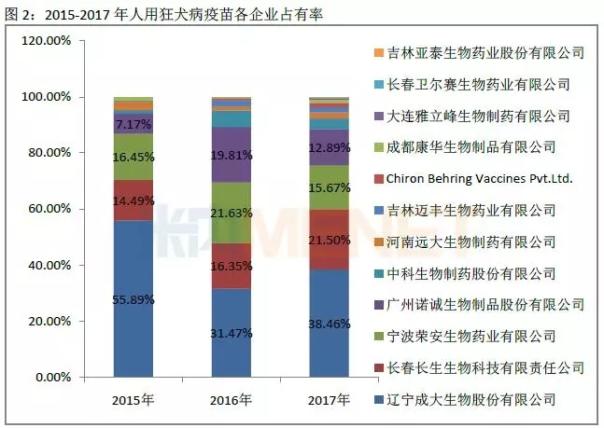
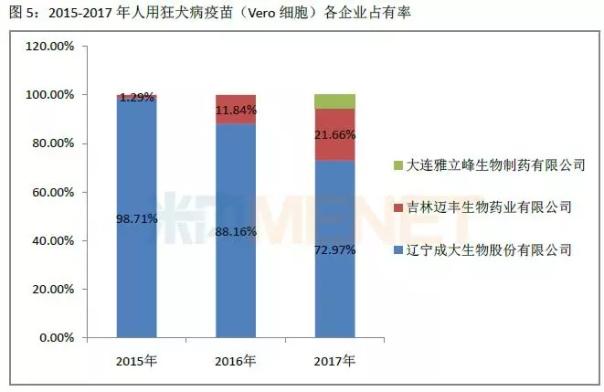
According to the data of the Chinese People's Procuratorate, the number of human rabies vaccines issued in 2015-2017 was 17.52 million, 12.68 million and 17.17 million, of which the largest bio-occupancy rate ranked first, 55.89 in 2015. %, stayed at 30% or more in 2016-2017. Followed by longevity organisms, the share in 2015-2016 remained between 14% and 17%, and rose to 21.05% in 2017.
(Note: According to the data of the Chinese Academy of Sciences)
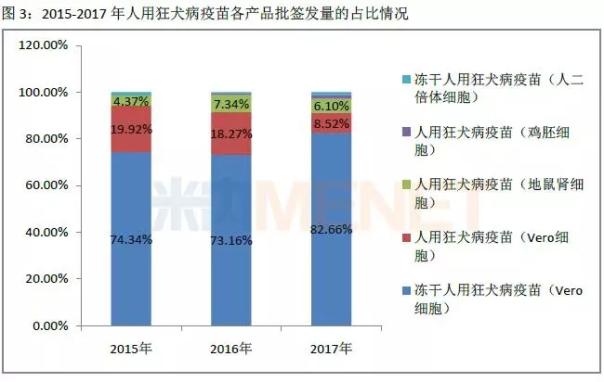
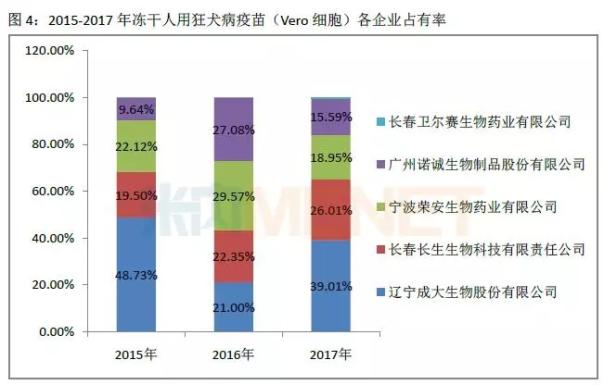
Subdivided into various products, the batch of rabies vaccine (Vero cells) for freeze-drying in 2015-2017 accounted for more than 70% of the total number of batches issued, followed by human rabies vaccine (Vero cells) 2015-2016 The number of batches issued was 18%-20%, and fell to 8.52% in 2017. In 2015-2017, the proportion of human rabies vaccine (hamster kidney cells), human rabies vaccine (chicken embryo cells), and freeze-dried human rabies vaccine (human diploid cells) was no more than 10%.
(Note: According to the data of the Chinese Academy of Sciences)
(Note: According to the data of the Chinese Academy of Sciences)
Recombinant human anti-rabies virus monoclonal antibody injection phase III clinical approval
(Source: Announcement of Listed Companies)
It is reported that the recombinant human anti-rabies virus monoclonal antibody injection is an independent innovation project of Huabei Pharmaceutical Subsidiary, and is listed as a major national special product of “Major New Drug Creationâ€. Its mechanism of action and indications are to use this product in combination with rabies vaccine to supplement the antibody blank in the active immunization process of rabies vaccine, which can directly neutralize rabies virus in vivo and play a passive immune role for rabies or other rabies. Passive immunization of patients with bites and scratches in toxic susceptible animals.
At present, the product has completed Phase I and Phase II clinical trials, and Phase III clinical trials have been approved by the Drug Clinical Trial. Up to now, the accumulated investment in research and development of this project is 740.187 million yuan.
At present, there is no recombinant anti-rabies virus monoclonal antibody market in China. In the international market, the recombinant anti-rabies virus monoclonal antibody injection developed by the Indian Institute of Serology was approved for marketing in India in December 2016; the Dutch Crucell and the Indian Zydus study The center has completed Phase II clinical.
North China Pharmaceutical: There is also a rabies virus monoclonal antibody combination preparation on the road
Figure 6: Review of recombinant human anti-rabies virus monoclonal antibody NM57S/NC08 injection preparation in North China Pharmaceutical
(Source: Mene Online MED China Drug Evaluation Database 2.0)
According to the data from the MED China Drug Evaluation Database 2.0, the rabies virus monoclonal antibody product of North China Pharmaceutical Group New Drug Research and Development Co., Ltd., in addition to the approved phase III clinical recombinant human anti-rabies virus monoclonal antibody injection, There is also a recombinant human anti-rabies virus monoclonal antibody NM57S/NC08 injection combination preparation in the review and approval. According to the annual report, the registration of this product is classified into Class 1 biological products, which are suitable for post-exposure prevention of rabies and upgraded products of human rabies immunoglobulin.
In addition, the recombinant anti-rabies virus humanized monoclonal antibody injection of Shenzhen Longrui Pharmaceutical Co., Ltd. was also approved in June 2017. The registration of this product is classified into two types of products.