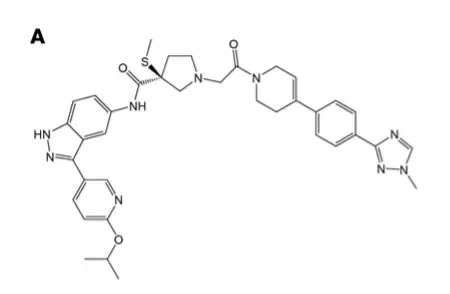
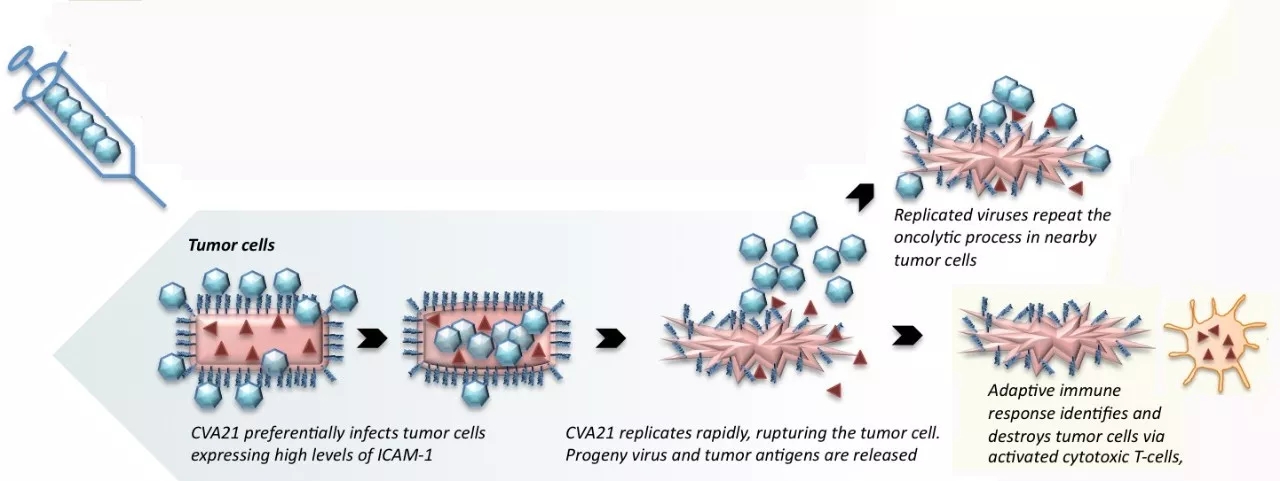
Summary of recent research progress in the field of cancer (03.05) March 05, 2018 Source: WuXi PharmaTech 1. First-line therapy! Lilly Breast Cancer New Drugs Approved by FDA Eli Lilly and Company has announced that the US FDA has approved the combination therapy of Verzenio (abemaciclib) and aromatase inhibitors developed by the company as an initial therapy for hormone receptor-positive (HR+), human epidermal growth. Menopausal patients with late-stage or metastatic breast cancer with factor receptor 2 negative (HER2-). Breast cancer is the most common female cancer in the world. About 30% of early breast cancer patients in the United States have metastases, and the survival rate of patients with metastatic breast cancer is significantly reduced. The 5-year survival rate of early breast cancer patients is 99%, while that of metastatic breast cancer patients is only 26%. Therefore, developing innovative therapies for advanced breast cancer is critical to saving patients' lives. Abemaciclib is an inhibitor of oral cyclin-dependent kinase (CDK) 4 and 6. In HR+, HER2- breast cancer cells, CDK4 and CDK6 promote phosphorylation of retinoblastoma protein (Rb) and promote cell cycle progression and cell proliferation. Abemaciclib is able to inhibit phosphorylation of Rb and block cells from the G1 phase to the S phase of the cell cycle, leading to cell senescence and apoptosis. In a randomized, double-blind, placebo-controlled phase 3 clinical trial called MONARCH 3, the progression-free survival (PFS) of advanced breast cancer patients receiving combination therapy with abemaciclib and aromatase inhibitors reached 28.2 months. Significantly higher than the combination of placebo and aromatase inhibitor (14.8 months). In patients with tumor size measurable, the objective response rate (ORR) was 55.4% in patients treated with a combination of abemaciclib and aromatase inhibitors. Of these, 52.1% were partial remission and 3.4% were complete remission. Patients who received a combination of placebo and aromatase inhibitors had an ORR of 40.2%, and all patients had partial remission. "FDA approval is an important milestone, indicating that combination therapy with abemaciclib and aromatase inhibitors can significantly reduce tumor size and delay progression of cancer in HR+, HER2-metastatic breast cancer patients." Baylor University Medical Center ( Dr. Joyce O'Shaughnessy, Chairman of the Breast Cancer Research Program at the Baylor University Medical Center, commented. 2. For the BRAF resistance, the new drug of Merck shows early efficacy Researchers at the University of North Carolina (UNC) Lineberger Comprehensive Cancer Center and other partner agencies reported that a new drug, Merck (MSD), has shown efficacy in early clinical trials of advanced melanoma and other drug-resistant cancers. Although targeted therapies have been approved for melanoma and lung cancer with specific mutations in the BRAF gene, most patients develop resistance to these treatments and cancers recur, with the most common cause being ERK reactivation. To overcome the resistance of cancer cells to anti-BRAF drugs, researchers at UNC Lineberger and other institutions have studied MK-8353 compounds to block ERK. MK-8353 is an oral, bioavailable, and selective potent ERK inhibitor that proportionally reduces the phosphorylated activation of ERK1, ERK2 and RSK proteins. The drug was clinically tested in Phase 1 in healthy volunteers (P07652) and advanced solid tumor patients (MK-8353-001). In the P07652 study conducted in the Netherlands, 48 ​​healthy volunteers were given a single oral dose of 10 to 400 mg of MK-8353. In the MK-8353-001 study, 26 cancer patients were orally administered 100 to 800 mg doses of MK-8353 twice daily. These studies analyzed the safety, tolerability, pharmacokinetics, pharmacodynamics and antitumor activity of the drug. ▲MK-8353 molecular structure (Source: "JCI Insight") MK-8353 was generally safe and well tolerated in all doses and no serious adverse events (SAE) occurred. Adverse events in P07652 included diarrhea (44%), fatigue (40%), nausea (32%) and rash (28%). Dose-limiting toxicity was observed in the 400 mg and 800 mg dose groups. Of the 15 patients who were evaluated for treatment remission in the MK-8353-001 study, 3 had partial remission, all of whom had BRAF V600 mutant melanoma. “ERK activates factors that promote cancer growth,†said Dr. Channing Der, a professor of pharmacology at the UNC School of Medicine. “ERK is very complex, and we still have very little understanding of it. But we can be sure it is necessary for cancer growth. Therefore, many inhibitors targeting ERK are approved or undergoing clinical evaluation." 3. $ 3 billion to create next-generation cell therapy, Kite and Sangamo reach global cooperation Recently, Gilead's Kite and Sangamo Therapeutics announced that the two companies have reached a global cooperation to develop the next generation of oncology in vitro cell therapy using Sangamo's zinc finger nuclease (ZFN) technology platform. Under the terms of the agreement, Sangamo will receive a $150 million advance payment and is eligible for up to $3.01 billion in potential payments (based on the development, regulatory and promotional milestones of more than 10 products using Sangamo technology). Sangamo is a company that specializes in turning breakthrough science into gene therapy that uses the company's leading platform technologies in genome editing, gene therapy, gene regulation and cell therapy to transform patients' lives. The company's ZFN platform is a powerful gene editing technology that can be used to specifically knock out genes or insert therapeutic genes into precise locations. Through this collaboration, Kite will use Sangamo's ZFN technology to modify genes to develop next-generation cell therapies for autologous and allogeneic treatment of different cancers. Allogeneic cell therapies from healthy donor cells or regenerative stem cells will provide a potential therapeutic option that can be performed directly at the tumor infusion center, thereby reducing the time of patient infusion. ▲Sangamo Therapeutics President and CEO Dr. Sandy Macrae introduces gene therapy at this year's WuXi PharmaTech Global Forum "This collaboration between Kite and Sangamo combines two leading platforms to jointly develop cell-therapies for best-in-class in oncology," said Dr. Sandy Macrae, President and CEO of Sangamo. "We are very happy." Kite is committed to driving innovation in this area and looks forward to working together to realize the full commitment of cell therapy in the field of cancer treatment." "As a tool for editing immune cells, the emergence of genetic editing promises to develop treatments that potentially improve safety, efficacy and efficiency," said Dr. John F. Milligan, President and CEO of Gilead. "We believe in Sangamo's zinc finger nucleic acid. Enzymes provide the best genetic editing platform, and we look forward to working with Sangamo to accelerate the development of next-generation autologous cell therapies and allogeneic gene therapy for cancer patients in a hospital setting." 4. Create oncolytic immunotherapy, Merck's acquisition of Viralytics Recently, Merck (MSD) and Viralytics announced that the two companies have signed a definitive agreement, through which Merck will acquire Viralytics for $394 million. Upon completion of the acquisition, Viralytics will become a wholly-owned subsidiary of Merck, and Merck will receive all the rights of Viralytics to study oncolytic immunotherapy CAVATAK. Viralytics is an Australian-based company developing cancer oncolytic immunotherapy. Its main candidate, CAVATAK®, is a proprietary formulation of the common cold coxsackievirus type A21 (CVA21), which binds to specific receptor proteins that are highly expressed on many types of cancer cells, through cell lysis and potentially against cancer cells. An immune response to kill local and metastatic cancer cells. This dual mechanism of action is known as oncolytic immunotherapy. Currently, CAVATAK is being evaluated as an intratumoral and intravenous formulation in a number of Phase 1 and Phase 2 clinical trials, including in combination with Merck's heavy anti-PD-1 therapy KEYTRUDA (pembrolizumab). According to an agreement announced by Viralytics and Merck's subsidiary in November 2015, a study is evaluating CAVATAK in combination with KEYTRUDA for the treatment of melanoma, prostate cancer, lung cancer and bladder cancer. ▲ How CAVATAK works (Source: Viralytics official website) “Viralytics complements our immuno-oncology strategy with an innate immune system that targets and kills cancer cells. We focus on rapidly advancing innovative monotherapy and synergistic combinations to help a wide range of cancer patients,†said Merck Research Laboratories. Dr. Roy Baynes, Vice President, Global Clinical Development Director and Chief Medical Officer, said: "As we continue to work to improve the long-term disease control and survival outcomes of cancer patients with the immune system, we are eager to further develop the technology of Viralytics." Reference materials: [1] Eli Lilly's Verzenio Picks Up Third Approved Indication [2] Merck's melanoma drug shows early promise for resistant cancers in PhI trial [3] Gilead's Kite Teams Up with Sangamo Therapeutics for Cancer Therapeutics in a $3.1 Billion+ Deal [4] Merck Drops $394 Million to Acquire Virus-Based Cancer Company Viralytics Original title: Summary of recent research progress in the field of cancer (No. 56) We're professional Gynecology Hysteroscopy
manufacturers and suppliers in China, specialized in providing high
quality medical instruments with reasonable price. We warmly welcome you
to buy or wholesale bulk Gynecology Hysteroscopy for sale here and get quotation from our factory. Gynecology Hysteroscopy,Hysteroscopy Instrument Set,Hysteroscopy Set,Outer Sheath Tonglu WANHE Medical Instrument Co., Ltd , https://www.tlvanhurhealth.com