Table 1 : Typical DNA yield and purity Sample type Sample volume DNA yield ( μg ) DNA purity ( OD260/280 ) Kit type blood blood Cultured cells Cultured cells 200 μl 500 μl 5 x 10 5 1 x 10 6 6.6 μg 16.5 μg 10.4 μg 22.0 μg 1.9 2.0 2.0 2.0 SV SV A high yield of purified viral DNA and RNA is achieved. It is possible to isolate viral nucleic acids from a wide range of concentrations (Figure 4). The MagNA Pure 96 system includes an internal quality control (IC) for each separation reaction. The IC is automatically transferred from the on-board IC tube into the lysis buffer in each well. The diluted parvovirus B19 plasmid was used as the IC in all 96 wells of one purification operation, and the negative serum was used as the sample material to examine the function. The separation of ICs is highly reproducible (Figure 5). The new MagNA Pure 96 DNA and Viral Nucleic Acid Kits provide fully automated and efficient separation of DNA and viral nucleic acids from a wide range of sample materials with high speed and high yield. Users can choose between several different methods depending on their specific needs. In addition, the sample can handle a wide range of sample sizes. Therefore, new kits will be a useful tool for laboratories with large workloads.
Our Surgical Dressing Products is good in quality and competitive in price. We are manufacturer and supplier Surgical Dressing Products following your specific requirement. We are looking forward to your E-mail and establishing cooperative relationship with you! We would provide professional Surgical Dressing with good services for you!
Surgical Dressing Cotton Products, Plaster Products, Non-Woven Products, Bandage Products Ningbo Cland Medical Instruments Co., Ltd. , https://www.ruipumedical.com

Figure 2 : Agarose gel on DNA isolated from blood (showing 20 copies). The DNA band at the 21 kb position shows high integrity of the isolated DNA . ( M = molecular weight marker III )
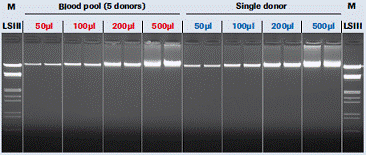
Figure 3 : Measureability. Agarose gel analysis of DNA isolated from different amounts of blood showed good quantifiability of the purification method. 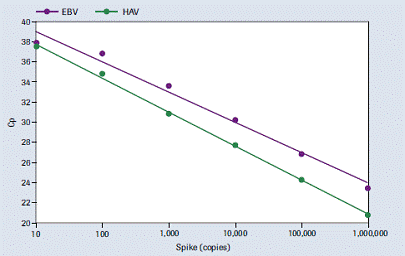
Figure 4 : Linear range. Separation of Epstein-Barr virus ( EBV ) DNA and Hepatitis A virus RNA at a concentration of 10-10 6 copies was added to each sample . 
Figure 5 : Internal control products. PCR analysis of parvovirus B19 internal controls isolated from all 96 wells of the treated sample cassette . 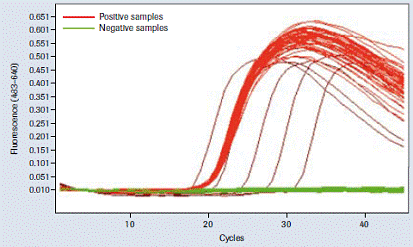
Figure 6 : Cross contamination test. Forty- eight positive and 48 negative samples were arranged in a checkerboard shape and processed for cross-contamination analysis. All negative samples were identified as " negative " and all positive samples and all controls showed the expected signal. The test was repeated 3 times and all gave the same results.
product Package size Numbering MagNA Pure 96 High-throughput Fully Automatic Nucleic Acid Isolation and Purification System Instruments, controls, software, all Accessories 05 195 322 001 MagNA Pure 96 DNA and Viral Nucleic Acid Small Capsule 3×192 purifications 05 467 497 001 MagNA Pure 96 DNA and Viral Nucleic Acid Bulk Kit 3×96 purifications 05 467 454 001 MagNA Pure 96 Cell RNA Small Capacity Kit 3×192 purifications 05 467 543 001 MagNA Pure 96 Cell RNA Bulk Kit 3×96 purifications 05 467 535 001
New MagNA Pure 96 DNA and Viral Nucleic Acid Kits:
A new solution that combines fast and high productivity
Michael Kirchgesser*, Agnes Aschenbrenner, Irene Huber, Sarah Müller, Heike Girgnhuber, Hubert Schuldlos, Anna Werner, Werner Malmberg, Robert Steiner, Martin Victor, Thomas Kirschbaum, Claudia Kappelsberger, Michael Knoll, and Peter Wenzig
Roche Applied Science, Pittsburgh, Germany
* Corresponding author: michael.
Foreword
MagNA Pure 96 is a new instrument that has recently entered the market: it is a fully automated, ultra-high-speed system that can be used to separate high-quality nucleic acids from high-speed nucleic acids in a variety of sample types. Automated purification of 96 samples with a sample capacity of up to 1 ml. The system uses an optimized kit with pre-packed ready-to-use reagents and a corresponding automated separation method. The kit's configuration is based on the long-standing magnetic bead technology that has been used successfully for different MagNA Pure systems for many years. The MagNA Pure 96 kit consists of reagent trays with different pre-packed sealed buffer containers and special bottles with magnetic powder.
All kits available are available in small (SV) and large (LV) sizes, depending on the amount of sample to be processed. This article describes the characteristics and performance of two MagNA Pure 96 DNA and viral nucleic acid kits and methods.
Figure 1 : Kit assembly.
Kit, reagent tray, magnetic bead bottle. Materials and Method
The new kits and methods were developed and tested using human EDTA plasma, citrate plasma, serum and whole blood, and cultured cells (K562 and HeLa) as sample materials.
Whole blood can be used for genomic DNA isolation without pretreatment. The cultured cell pellets are suspended in PBS prior to use.
In the case of viral nucleic acid isolation, different amounts of DNA virus (cytomegalovirus, Epstein-Barr virus, parvovirus B19) and RNA virus (Hepatitis A virus, Influenza A H1N1 influenza virus) are added to the sample before use. The sample was then pipetted into a MagNA Pure 96 process cartridge on a MagNA Pure 96 instrument. All samples were tested at least 8 fold replication. The elution volume of the purified nucleic acid was set to 50 μl, 100 μl or 200 μl according to the experimental needs. The eluate was analyzed on an LightCycler® 480 instrument using absorbance (OD) assay, agarose gel electrophoresis, and/or parameter-specific quantitative real-time PCR and RT-PCR analysis. The amplification reaction was established using 15 μl of each of the mixed mother liquors and 5 μl of the respective eluates for 45 cycles.
Results and discussion
General characteristics
The composition of the kit: pre-packed reagent containers arranged in the reagent tray for easy loading; separate bottles containing magnetic beads (MGP) suspension (Figure 1). The reagent tray and bottle are placed in their respective positions in the instrument drawer before they are run. The container can be easily resealed after use and stored for later operation if necessary. The MGP bottle has a special cap that automatically reseals after the needle of the instrument has passed. All components are stable at room temperature.
MagNA Pure 96 DNA and viral nucleic acid small-capacity specifications are used to isolate genomic DNA from 200 μl of whole blood, or from 5 × 10 5 cultured cells. It can also isolate viral DNA or RNA from 200 μl of plasma, serum or whole blood. The kit includes 3 pre-filled reagent containers, each of which can perform up to 192 reactions (ie, 576 purifications per kit).
MagNA Pure 96 DNA viral nucleic acid and a kit for large capacities of genomic DNA isolated from whole blood or 500μl or 1000μl from 10 6 cultured cells. It can separate viral DNA or RNA from 500 μl plasma, serum or whole blood, or from 1000 μl plasma or serum. The kit includes 3 pre-filled reagent containers, each of which can perform up to 96 reactions (500 μl) or 48 reactions (1000 μl) (ie, 288 or 144 purifications per kit).
If required, the kit can be used for different optimization methods, including external dissolution methods, allowing for virus inactivation outside of the MagNA Pure 96 instrument. For all methods, nucleic acids can be conveniently eluted from samples of different capacities, and the different capacities of the samples can be selected by the user.
Genomic DNA
Isolation of genomic DNA and subsequent analysis revealed high reproducibility, yield, integrity and purity. According to agarose gel analysis, the DNA fragments were significantly larger than 20 kb on average (Fig. 2).
Table 1 summarizes typical DNA yields and purity. Please note that the yield is significantly dependent on the number of blood cells, so the change may be doubled.
As shown by the dilution experiments prior to PCR performed in the LightCycler® 480 instrument (data not shown), DNA was also shown to be free of PCR inhibitors.
The measurability of the procedure was tested with different amounts of blood and samples. The result is shown in Figure 3.
Viral nucleic acid
Linear range
Internal quality control and repeatability
Cross contamination test
These tests were conducted to examine the extent of possible inter-well contamination on the MagNA Pure 96 instrument. Parvovirus B19 was selected as a sensitive parameter for these assays; the detection limit was approximately 2.5 copies per PCR. Then 5 × 10 7 copies / ml (= detection limit of 106 times or more) were added to the plasma sample, a method with a large capacity (500 l samples) and elution volume 50μl treatment instrument MagNA Pure 96.
Three "checkerboard" runs were performed (every 48 positive spiked plasma samples and 48 negative unloaded plasma samples were arranged in a checkerboard pattern). The eluate was analyzed on a LightCycler ® 480 instrument. No cross-contamination was found under these conditions (Figure 6).
" Plasma " and " General " methods
During the optimization of the method, it was found that some sample materials (ie, serum, whole blood, and citrate plasma) gave better results in a different workflow than the "standard" material EDTA plasma. Therefore, in addition to the "Plasma Method of Operation", another "general operation method" is provided which enables better results for these sample types (up to 5 cross thresholds). The term "general method of operation" was chosen because these methods are superior to "plasma methods" for serum, whole blood, and citrate plasma; and for EDTA plasma, at least for the virus tested (hepatitis A virus, Influenza A (H1N1, Epstein-Barr virus, cytomegalovirus) is equivalent to the "method of operation for plasma". Since the results may vary with individual experimental equipment and the parameters being tested, different methods are available in the instrument's method menu to allow the user to choose the best solution for the application.
in conclusion