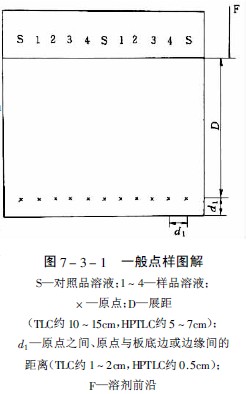
Thin layer chromatography Spotting method The spotting is sampled on the thin layer plate, usually a dot, and the base line is 2.0 cm from the bottom edge. The sample diameter and the distance between the points are the same as the paper chromatography method. It is advisable to regard the spread of the spots so as not to affect the detection. Care must be taken not to damage the surface of the sheet when spotting. The spotting diameter is not more than 5mm, and the spotting distance is generally 1~1.5cm. The solubility of the sample in the solvent is large and the origin will be a hollow ring - ring chromatography effect. Therefore, a solvent with a relatively small solubility to the components should be selected when formulating the sample solution. The spotting method is a bit like a spot and a strip. How to improve the efficiency of spotting? Spotting is the main source of quantitative error in TLC. Experiments have shown that quantitative capillaries are more suitable for smaller volume spotting; microinjectors are more suitable for larger volume spotting. This is mainly because the micro-syringe is greatly affected by small bubbles and solution back crawling. In order to avoid spotting errors between different quantitative capillaries, it is recommended to use the same quantitative capillary on a thin plate. However, it should be noted that when replacing the sample, the capillary should be cleaned with a super-bo or a different polar solvent. When preparing the sample, the viscosity of the solvent should not be too high to facilitate spotting; if the boiling point of the solvent is too low, the injection volume is apt to change, and too high will change the composition of the developing agent; if the solubility of the sample is too high, the sample will be hollow. Commonly used solvents are methanol, ethanol, **. The origin of the classical TLC sample is generally 3 mm in diameter and 1 - 2 cm in pitch. The bottom margin is 1.5 cm. The origin of the HPLC sample is generally 1 mm in diameter and 5 mm in pitch. The bottom margin is 1 cm. Different solvents have different elution ability to the sample, so at the same time as the sample is sampled, the sample starts to expand in a ring shape. If the solubility of the sample in the solvent is large, the origin will become a hollow ring. Kaiser calls this phenomenon a "point." Sample ring chromatography effect." This effect has an adverse effect on the subsequent linear development. Therefore, a solvent with a relatively small solubility of the components should be used when preparing the sample solution; the viscosity of the solvent should not be too high, so as to facilitate the spotting, the boiling point of the solvent should be moderate, and the boiling point is too low. Since the volatilization will change the concentration of the sample solution, resulting in a large error, the boiling point is too high, and the solvent of the sample solution will remain at the origin, resulting in a change in the selectivity of the developing agent, especially when the solvent of the sample solution differs greatly from the polarity of the developing agent. To be obvious, the most commonly used solvents are methanol and ethanol, and sometimes acetone. After the spotting, the solvent must be completely removed before unfolding, but high temperature heating should be avoided to avoid changing the properties of the component to be tested. (Changsha Dezhirui Instrument Technology Co., Ltd. quantitative capillary) The selection method, the amount of spotting and the selection of the spotting device are determined by the purpose of the analysis, the concentration of the sample solution, and the detection sensitivity of the substance to be tested. The typical thin layer of spotting volume is generally 1-5μl, the high-efficiency thin layer is 100-500nl, and the concentration of sample solution is generally in the range of 0.01%-1.00%. Too much spotting will cause the origin to be “overloaded†and the developing agent will produce “circumferenceâ€. The phenomenon causes the spots to be tailed or overlapped, so that the scan peak shape is asymmetrical or not separated by the baseline, and a nonlinear calibration curve is obtained due to the overload, which seriously affects the quantitative result and reduces the accuracy. The diameter of the origin of the classic thin layer should not exceed 5mm at most, generally 3mm is more suitable, the diameter of the high-efficiency thin layer origin is about 1-2mm, avoiding multiple times as much as possible, and the original diameter is too large to reduce the resolution and resolution. The starting line of the classic thin layer is about 1.5cm from the bottom edge, the high-efficiency thin layer is 1cm, the former is 10-15cm, and the latter is 5-7cm. When the multi-developer is difficult to be separated, pay attention to the origin and development. The distance of the agent should be consistent each time, otherwise the separation will fail due to the difference in the initial behavior of the chromatographic separation. The point-to-point spacing is 1-2cm for the classic thin layer and 0.5cm for the high-efficiency thin layer. When the thin layer scanner with the automatic wrap function, the distance between the spots must be very accurate to correctly scan the stains of each line. A good measurement result is obtained, and Fig. 7-3-1 is a general spotting diagram. Before spotting, the thin layer board should be inspected for damage or contamination under the sunlight and ultraviolet light. After selecting the qualified thin layer board, it is decided to use the spot shape or the strip shape. To this end, CAMAG Switzerland produces a range of spotting equipment for use, these devices are only suitable for hard boards. The following are commonly used spotting methods and equipment. (Changsha Dezhirui Instrument Technology Co., Ltd. quantitative capillary) (1) Dot-like spotting If only for qualitative purposes, the sample solution is placed at a distance of about 2 cm from the bottom edge of the thin layer with a capillary or micro-syringe having a flat inner diameter of about 0.5 mm, and the spot diameter is not more than 5 mm. 1-1.5cm can be. (2) Strip-like spotting When the sample solution is bulky and dilute, the CAMAG Linoamt IV automatic spotting device is used for strip-like spotting. The range of spotting for quantitative analysis is 1-99μl, and the range of preparative separation is 5-490μl. When using the same standard solution, the standard solution can be automatically placed on the same standard solution, so it is quick and easy to use. The processor sequence operation is simple and easy. When in use, the sample solution is sucked into the micro-syringe. The spotter does not touch the thin layer. Instead, the solution of the needle tip of the syringe is blown onto the thin layer with nitrogen. The thin plate moves at a constant speed under the needle to a narrow band of 0-199 mm. . The spotted spotted spot is not only sharper than the spotted spot, but also precise and accurate, providing the best conditions for quantitative analysis. (3) Automatic spotting Automatic TLC Sampler III (ATS III) automatic spotting device combines the most advanced modern electronic and mechanical technology, can be spotted or strip shaped, using advanced computer programming control, flexible and versatile, The spotting amount is 10nl-50μl, and the spotting specification can be set freely. It can be applied to one-way, two-way, circular or centripetal chromatography. The highly automated spotting makes the quantitative analysis result accurate. Spotting parameters can be used with the Scanner II scanner's CATS scan analysis software to make quantitative analysis work highly automated. (4) Contact spotting When the viscosity of the sample solution is large or the spot volume is more than 100 μl, it is difficult to spot by capillary. The contact spot method designed by Fenimore is suitable for the spotting of such samples. Clarke Contact made in the USA. Spotter is the contact point sampler, see Figure 7-3-2. A polyfluoride film (a) coated with a hydrophobic substance is covered over the concave block with the suction holes, and the film is recessed (b) when the pressure is evacuated, and the sample solution is spotted at the groove ( c), then most of the solvent (d) is purged with nitrogen, and the concentrated sample droplets are contacted with the adsorbent layer of the high efficiency lamina (e), at which point the sample is quantitatively transferred to the thin layer. Fig. 7-3-9 is a schematic diagram of FE-TLC. The gas from the high pressure gas cylinder 1 is pressed to 7.0-40 MPa by the electric diaphragm compressor 6, and the compressor and the micro extractor 12 are kept at a constant temperature in the thermostat. The volume of the extractor was 2 ml, and the sample to be extracted was placed therein. The valve 11 is closed first, and the pressure in the high pressure extractor reaches the extraction pressure, and the extraction process is opened after being balanced. The supercritical fluid is ejected through a capillary. Since the gas flow pressure drops to atmospheric pressure, the extracted extract is separated and adsorbed on the thin plate 13, and the distance between the thin plate and the capillary outlet is 1-5 mm. After the spotting, follow the thin layer conventional method. By stepwise increasing the extraction pressure, different components can be proposed step by step. (Changsha Dezhirui Instrument Technology Co., Ltd. quantitative capillary) Universal Surgical Pack,Sterile Universal Pack,Disposable Universal Pack,Disposable Sterile Universal Pack Xinxiang Huaxi Sanitary Materials Co., Ltd. , https://www.huaximedical.com
For quantification, there are two types of constant volume tubes for capillary action: one is 0.5, 1, 2, 3, 4, and 5 μl of the Capsules (Microcaps), and the other is 100 and 200 nl. Platinum-rhodium alloy quantitative capillary (Nanopipette); syringe-type variable volume spotter has 50-230nl Nano-Applicator and 0.5-2.3μl Micro-Applicator, Used for spotting of thin layers of bonded phase that require volume adjustment and no capillary action.
The capillary micro-spot is mounted on the magnetic spotting head of the manual Nanomat type spotting device, and the spotting head is mounted on the spring. Press this point and the capillary contacts the surface of the thin layer with a constant pressure. The distance between points is controlled by an E-shaped nicking device with an accurate #44 spacing. The CAMAG Nanomat III manual spotting device is suitable for ordinary or high-efficiency thin-layer plates. The sampling volume is accurate, the sample origin is small, and the origin spacing is accurately positioned. The nano spot applicator and micro spot applicator are controlled by a micrometer and can also be used with the Nanomat type spotting device to make the spotting position accurate.
The electric Nanomat III type spotting device is fully suitable for use with the above various spotters, and it can also automatically control the number of spotting, the dwell time of the spotter on the thin, and the speed at which the spotter contacts the thin layer. . It is also possible to avoid uneven force when holding a capillary tube, and the pressure is constant due to mechanical control of the contact surface. In addition, there are thin layer spotting devices that can be used for annular deployment and centripetal deployment. 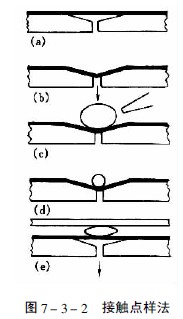
In 1988, Kaiser compared the above various sampling methods. The advantages and disadvantages are summarized in Table 7-3-1 and Figure 7-3-3. 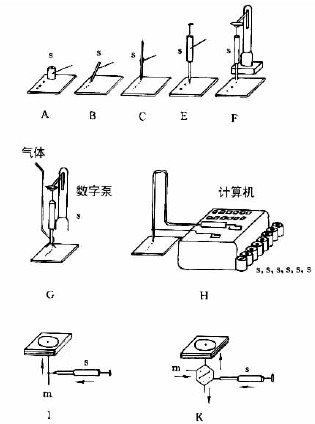
The error source for loading with a tubular spotter is due to the damage of the thin surface of the sample. The "wall climbing" effect of the solution causes the deposition of the outer wall sample, the memory effect of the sample, and the tiny bubbles in the tube. See Figure 7-3. -5.
The size of the spot error and the solubility of the sample, the surface tension of the solution, the viscosity of the solvent, the volatility and polarity, the time taken for the spotting, the quality of the laminate (grain size, uniformity, thickness of the layer, adhesive) The nature of the matter) and so on.
(Changsha Dezhirui Instrument Technology Co., Ltd. quantitative capillary) 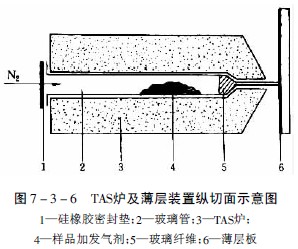
This rapid and rapid loading method has become an important means for the separation and identification of natural compounds and synthetic polymer compounds. Shimomura et al. applied this method in the study of the identification of traditional Chinese medicine thick flutter. Only a small amount of sample can be used to identify honokiol. Hashimoto said that the optical isomer of ephedrine was separated by this method. The alkali test was negative, demonstrating that the evaporation of ephedrine was complete after heating. Jolliffe et al. will use some alkaloid drugs, such as aconite, betel nut, belladonna, cinchona, ergot, mandala, ephedra, fairy, poppy, Rauvolfia, and hibiscus. The micro-extraction method, the volatile components escaping directly on the thin layer, were developed with five different developing agents, and Rhodamine B and cream yellow were used as reference materials, and the isolated alkaloids were identified after color development. Cheml et al. volatilized the alkaloids in the bark of Cinchona to a silica gel plate at a furnace temperature of 300 ° C, and developed seven alkaloids such as Cinchonine after development. Van Meer et al. successfully separated the valerin and isoorbitin from the roots of Valeriana by this method. Liptak et al. used this method to test fennel, chamomile and peppermint and their essential oils. 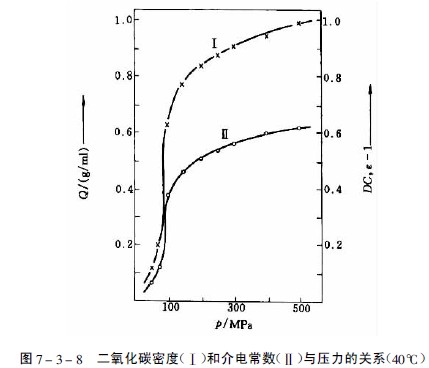

Stahl studied the relationship between the extractability of different compounds and the chemical structure using carbon dioxide as the extraction gas. The experimental results show that hydrocarbons and lipophilic compounds, such as esters, ethers, lactones and oxides, can be extracted at a pressure of 7.0-10.0 MPa, and the introduction of -OH or -COOH groups in the structure makes extraction difficult for polyhydric phenols. Polyphenolic acids, sugars and amino acids cannot be proposed at 500 atm. 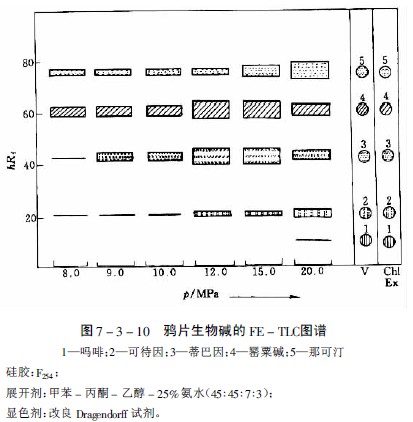
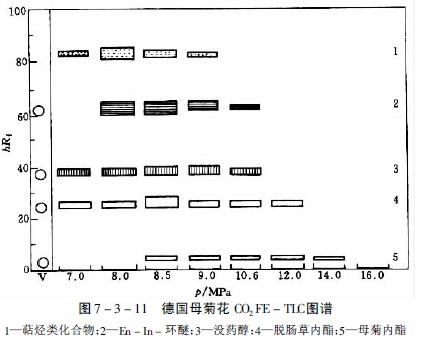
Table 7-3-1 Comparison of various spotting methods
In Figure 7-3-3, AF is the most commonly used capillary or syringe spotting: A—a thin-walled glass tube that is several millimeters long; B—a fused silica tube with a length of 5-60 mm and an inner diameter of 0.2-0.05 mm. Manipulator operation; C—Platinum-bismuth alloy tube (100nl, 200nl) sealed in glass tube, can be accurately sampled, and the biological sample can be cleaned by flame after spotting, see Figure 7-3-4; E-micro syringe, mechanical control , the contact plate surface is spotted; F—same E, with stepper motor control operation; G—with E, the sample solution is sprayed onto the thin layer plate with gas; H—same E, completely controlled by computer; I, K , L - the pump system is used for high pressure planar liquid chromatography (HPPLC); M - is Fenimore's solid sample toroidal transfer device.
(5) Special spotting technology Extraction is the first step before the thin layer separation of plant components. The extraction of the substance to be tested is usually carried out with a suitable solvent. However, this method is troublesome and requires a large amount of sample. The thermally unstable component is easily destroyed, so a method of directly spotting the sample without extraction has been studied.
1. Heat micro-extraction method Half a century ago, some drugs were sublimed, and the crystal form, melting point and physical and chemical properties of the sublimate were identified. In the simplest case, just a few milligrams of the crude drug powder is heated on a micro-slide and the sublimate is collected onto another slide 1 mm away from the sample slide. In 1968, Stahl used thermal micro-separation transfer technology in combination with thin-layer chromatography, which is called “hot micro-extraction methodâ€. This method has the advantages of greatly shortening the time for extracting volatile components from crude drugs, simplifying the extraction method and reducing the extraction method. The disadvantage of the amount of sample is that it is not suitable for non-volatile ingredients.
Place the plant powder sample in a thinned glass tube at one end and simultaneously place a suitable additive containing a certain amount of water to maintain a continuous flow of water during the heating process (in addition to the aqueous molecular sieve for neutral gas in the TAS method) In addition to the agent, an acid gas generating agent such as malonic acid can be used to generate carbon dioxide and acetic acid at 180 ° C, so that the alkaline volatile component in the sample can be retained as salt without escaping, and the acidic volatile component is Acid gas is transferred to the thin layer; vice versa, an alkaline gas generating agent, such as calcium chloride containing ammonia, can be used to transfer the alkaline component of the sample to the thin layer with ammonia gas, and one end of the tube is tightly packed with a silicone rubber film. It is closed and placed on a TAS furnace at a certain temperature. The device is shown in Figure 7-3-6.
Heated to 220 ° C, the volatile components vaporized, and escaped from the capillary port at one end of the glass tube, while the nitrogen flow in the tube at the same time with 30ml / min nitrogen flow to help the volatile components escape, the escaped gasification component points added to the outlet On a thin layer of 1 mm, after the sample is completed, it can be obtained by a conventional method to obtain a chromatogram of the hot fraction.
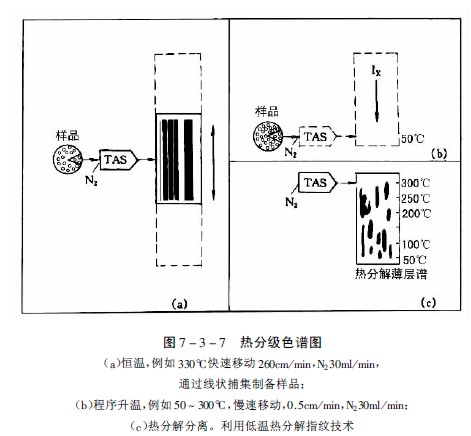
Stahl and Lott developed this method and designed a thin-layer plate device that can move laterally at a certain speed, so that volatile components or thermal decomposition products after heating can be attached to the moving thin-layer plate starting line, and the heating method can be constant temperature or program. The temperature is raised so that the characteristic spectra of various samples can be obtained, and the improved method is called thermal fractionation chromatography. Figure 7-3-7 is a characteristic map obtained with a commercially available "TASOMAT" device.
The composition of proprietary Chinese medicines is complex, and the TLC chromatographic identification methods of Chinese patent medicines contained in the Pharmacopoeia are all compared with single-flavored medicinal materials or single components after solvent extraction. Zeng Meiyi and others tried to identify Chinese patent medicines by hot micro-transfer method. The method is as follows:
Treatment of the prescription preparation: the honey pill is mixed with the same amount of silica gel 5; the tablet is ground into fine powder; the capsule and the powder are not treated; the oil agent can be spotted on the silica gel plate, and then the silica gel is scraped off with oil.
Thermal micro-transfer conditions The sample was placed in a thin tube wound with aluminum foil, and an aqueous molecular sieve was added thereto, placed in a tube, and heated at 220-225 ° C for 60-90 s. Volatile, thermally decomposing or sublimating components in the sample are transferred directly to the thin layer.
Position with anisaldehyde reagent or with fluorimetry.
The sample dosage is 10-16 mg for the prescription and 4-8 mg for the single dose. The samples tested were Guanxin Suhe Pill, Compound Danshen Tablet, QiLi San, Yinqiao Jiedu Pill, Liuwei Dihuang Pill, etc., and the single-flavored medicine was successfully explored. The advantage is that it does not need to go through complicated Processing can be used to obtain a chromatogram that can be identified. In addition, the method can also be applied to semi-quantitative analysis of small quantities of crude drugs, micro-preparation of crude drug ingredients, and fingerprint identification of crude drugs.
2. Fluid extraction method
The gas in the phase region above the critical pressure and the critical temperature is a supercritical fluid, and the density, dielectric constant and solvent-like properties of the gas are significantly increased, as shown in Fig. 7-3-8. The solubility of a substance in a supercritical fluid is greatly increased by the enhanced interaction between the compressed gas and the solute molecules.
Changing the temperature and pressure can change the characteristics of the supercritical fluid, so it is possible to extract different polar components from the sample under certain temperature and pressure conditions. This method is combined with thin layer chromatography, which means that the fluid extraction is thin. Layer chromatography (Fluid extraction-TLC, FE-TLC).
The gases used in this extraction process are carbon dioxide, nitrous oxide, ethylene, trifluoromethane, carbon hexafluoride, nitrogen, argon, and the like. The physical parameters of the various gases at the critical point are listed in Table 7-3-2. Among them, carbon dioxide is the most commonly used gas.
Table 7-3-2 Physical parameters of several gases
When studying the extractability of 27 alkaloids such as Rauvolfia, Periwinkle, Poppy, Quinine and Ephedra, nitrous oxide was selected as a suitable extraction gas, but the extraction ability varied with alkaloids. For example, when separating the main alkaloids in opium, papaverine and narcotine are easy to propose at about 8PMa, and thebaine and codeine are more difficult to propose, while morphine only proposes a very small amount even at a pressure of 20 MPa. A thin layer chromatography was extracted as shown in Figure 7-3-10 and Figure 7-3-11.
Maternal lactone is an active ingredient of German chrysanthemum, which is highly susceptible to thermal decomposition and produces blue Jerusalem artichoke, which cannot be obtained by steam distillation. With solvent extraction, the yield is low. For example, by extraction with carbon dioxide supercritical fluid, the parent chrysanthemum and volatile oxygen compounds can be obtained by partial extraction, and the mother chrysanthemum crystal can be obtained by silica gel column chromatography.